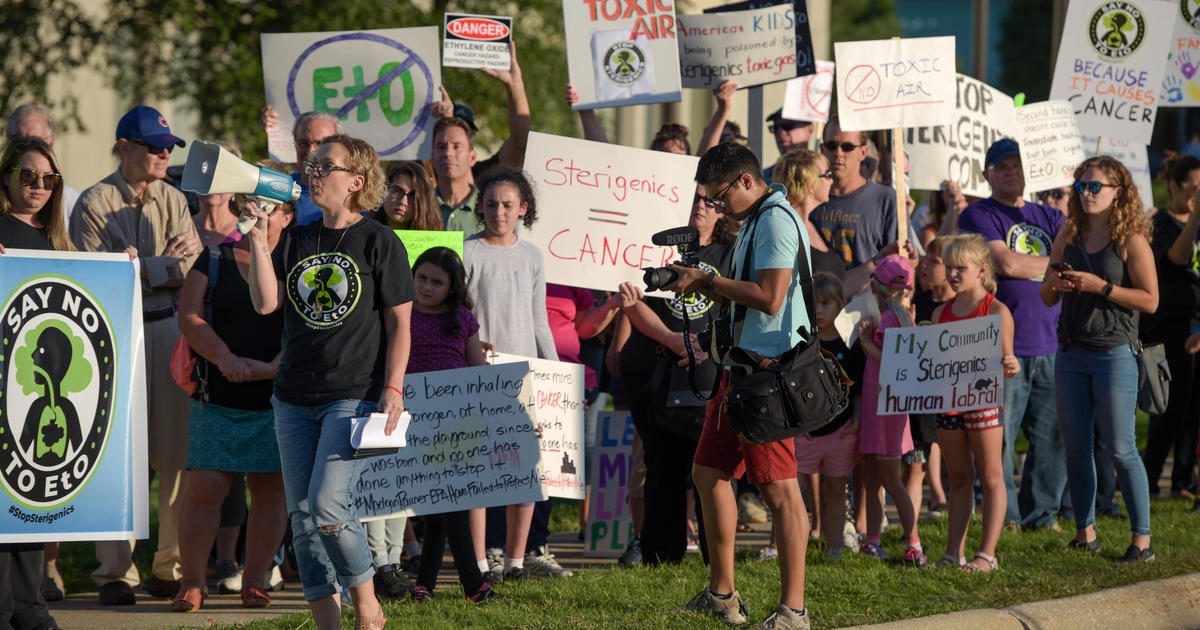
New safety rules ahead for toxic gas used to sterilize medical devices
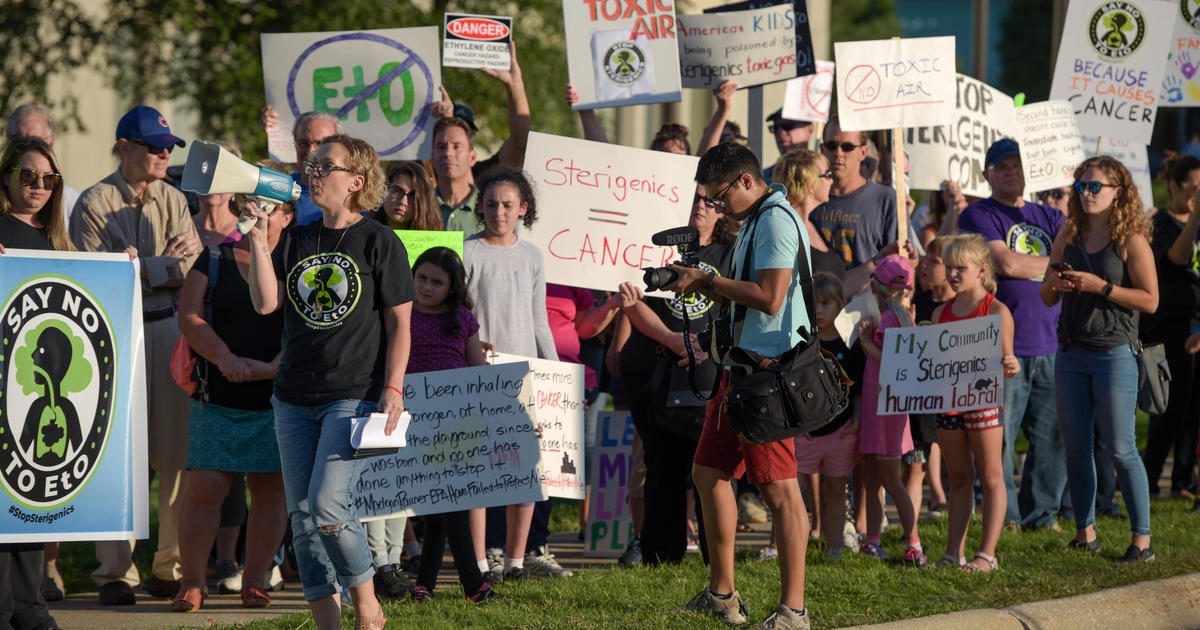
Over the past two years, Madeline Beal has heard frustration and even bewilderment during public meetings about ethylene oxide, a cancer-causing gas that is used to sterilize half of the medical devices in the U.S.
Beal, senior risk communication adviser for the Environmental Protection Agency, has fielded questions about why the agency took so long to alert people who live near facilities that emit the chemical about unusually high amounts of the carcinogenic gas in their neighborhoods. Residents asked why the EPA couldn’t close those facilities, and they wanted to know how many people had developed cancer from their exposure.
“If you’re upset by the information you’re hearing tonight, if you’re angry, if it scares you to think about risk to your family, those are totally reasonable responses,” Beal told an audience in Laredo, Texas, in September 2022. “We think the risk levels near this facility are too high.”
warnings about cancer risk from the federal government and media reports, an awareness that has also spawned protests and lawsuits alleging medical harm.
The EPA is expected to meet a March 1 court-ordered deadline to finalize tighter safety rules around how the toxic gas is used. The proposed changes come in the wake of a 2016 agency report that found that long-term exposure to ethylene oxide is more dangerous than was previously thought.
But the anticipated final rules — the agency’s first regulatory update on ethylene oxide emissions in more than a decade — are expected to face pushback. Medical device-makers worry stricter regulation will increase costs and may put patients at higher risk of infection from devices, ranging from surgical kits to catheters, due to deficient sterilization. The new rules are also not likely to satisfy the concerns of environmentalists or members of the public, who already have expressed frustration about how long it took the federal government to sound the alarm.
“We have been breathing this air for 40 years,” said Connie Waller, 70, who lives with her husband, David, 75, within two miles of such a sterilizing plant in Covington, Georgia, east of Atlanta. “The only way to stop these chemicals is to hit them in their pocketbook, to get their attention.”
The EPA says data shows that long-term exposure to ethylene oxide can increase the risk of breast cancer and cancers of the white blood cells, such as non-Hodgkin lymphoma, myeloma and lymphocytic leukemia. It can irritate the eyes, nose, throat and lungs, and has been linked to damage to the brain and nervous and reproductive systems. Children are potentially more vulnerable, as are workers routinely exposed to the chemical, EPA officials say. The agency calculates the risk based on how much of the gas is in the air or near the sterilizing facility, the distance a person is from the plant and how long the person is exposed.
Waller said she was diagnosed with breast cancer in 2004 and that her husband was found to have non-Hodgkin lymphoma eight years later.
A 2022 study of communities living near a sterilization facility in Laredo found the rates of acute lymphocytic leukemia and breast cancer were statistically significant, greater than expected compared with statewide rates.
Beal, the EPA risk adviser, who regularly meets with community members, acknowledges the public’s concerns. “We don’t think it’s OK for you to be at increased risk from something that you have no control over, that’s near your house,” she said. “We are working as fast as we can to get that risk reduced with the powers that we have available to us.”
In the meantime, local and state governments and industry groups have scrambled to defuse public outcry.
Hundreds of personal injury cases have been filed in communities near sterilizing plants. In 2020, New Mexico’s then-attorney general filed a lawsuit against a plant in Santa Teresa, and that case is ongoing. In a case that settled last year in suburban Atlanta, a company agreed to pay $35 million to 79 people who alleged ethylene oxide used at the plant caused cancer and other injuries.
In Cook County, Illinois, a jury in 2022 awarded $363 million to a woman who alleged exposure to ethylene oxide gas led to her breast cancer diagnosis. But, in another Illinois case, a jury ruled that the sterilizing company was not liable for a woman’s blood cancer claim.
Greg Crist, chief advocacy officer for the Advanced Medical Technology Association, a medical device trade group that says ethylene oxide is an effective and reliable sterilant, attributes the spate of lawsuits to the litigious nature of trial attorneys.
“If they smell blood in the water, they’ll go after it,” Crist said.
Most states have at least one sterilizing plant. According to the EPA, a handful, like California and North Carolina, have gone further than the agency and the federal Clean Air Act to regulate ethylene oxide emissions. After a media and political firestorm raised awareness about the metro Atlanta facilities, Georgia started requiring sterilizing plants that use the gas to report all leaks.
The proposed rules the EPA is set to finalize would set lower emissions limits for chemical plants and commercial sterilizers and increase some safety requirements for workers within these facilities. The agency is expected to set an 18-month deadline for commercial sterilizers to come into compliance with the emissions rules.
That would help at facilities that “cut corners,” with lax pollution controls that allow emissions of the gas into nearby communities, said Richard Peltier, a professor of environmental health sciences at the University of Massachusetts-Amherst. Stronger regulation also prevents the plants from remaining under the radar. “One of the dirty secrets is that a lot of it is self-regulated or self-policed,” Peltier added.
But the proposed rules did not include protections for workers at off-site warehouses that store sterilized products, which can continue to emit ethylene oxide. They also did not require air testing around the facilities, prompting debate about how effective they would be in protecting the health of nearby residents.
Industry officials also don’t expect an alternative that is as broadly effective as ethylene oxide to be developed anytime soon, though they support researching other methods. Current alternatives include steam, radiation and hydrogen peroxide vapor.
Increasing the use of alternatives can reduce industry dependence on “the crutch of ethylene oxide,” said Darya Minovi, senior analyst with the Union of Concerned Scientists, an advocacy group.
But meeting the new guidelines will be disruptive to the industry, Crist said. He estimates companies will spend upward of $500 million to comply with the new EPA rules and could struggle to meet the agency’s 18-month timetable. Sterilization companies will also have difficulty adjusting to new rules on how workers handle the gas without a dip in efficiency, Crist said.
The Food and Drug Administration, which regulates drugs and medical devices, is also watching the regulatory moves closely and worries the updated emissions rule could “present some unique challenges” if implemented as proposed, said Audra Harrison, an FDA spokesperson. “The FDA is concerned about the rule’s effects on the availability of medical devices,” she added.
Other groups, like the American Chemistry Council and the Texas Commission on Environmental Quality, the state’s environmental agency, assert that ethylene oxide use isn’t as dangerous as the EPA says. The EPA’s toxicity assessment has “severe flaws” and is “overly conservative,” the council said in an emailed statement. Texas, which has several sterilizing plants, has said ethylene oxide isn’t as high a cancer risk as the agency claims, an assessment that the EPA has rejected.
Tracey Woodruff, a researcher at the University of California-San Francisco who previously worked at the EPA, said it can be hard for the agency to keep up with regulating chemicals like ethylene oxide because of constrained resources, the technical complications of rulemaking and industry lobbying.
But she’s hopeful the EPA can strike a balance between its desire to reduce exposure and the desire of the FDA not to disrupt medical device sterilization. And scrutiny can also help the device sterilization industry think outside the box.
“We continue to discover these chemicals that we’ve already been exposed to were toxic, and we have high exposures,” she said. “Regulation is an innovation forcer.”
KFF Health News, formerly known as Kaiser Health News (KHN), is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling and journalism.
Source: cbsnews.com